Products
Alginates
MedVance® Alginate Dressing
CuraVance® Silver Alginate
CuraVance® Silver Alginate is indicated for the management of moderate to heavily exuding chronic or acute wounds, including venous leg ulcers, diabetic foot ulcers, pressure injuries, donor sites, partial and full thickness burns and abrasions.
Antibacterial Dressings
CuraVance® Silver Alginate
CuraVance® Silver Alginate antibacterial calcium alginate dressing is rich in highly absorbent soft gelling mannuronic acid. The non-woven pad format manages exuding wounds, and the rope is appropriate for filling cavities and indurations
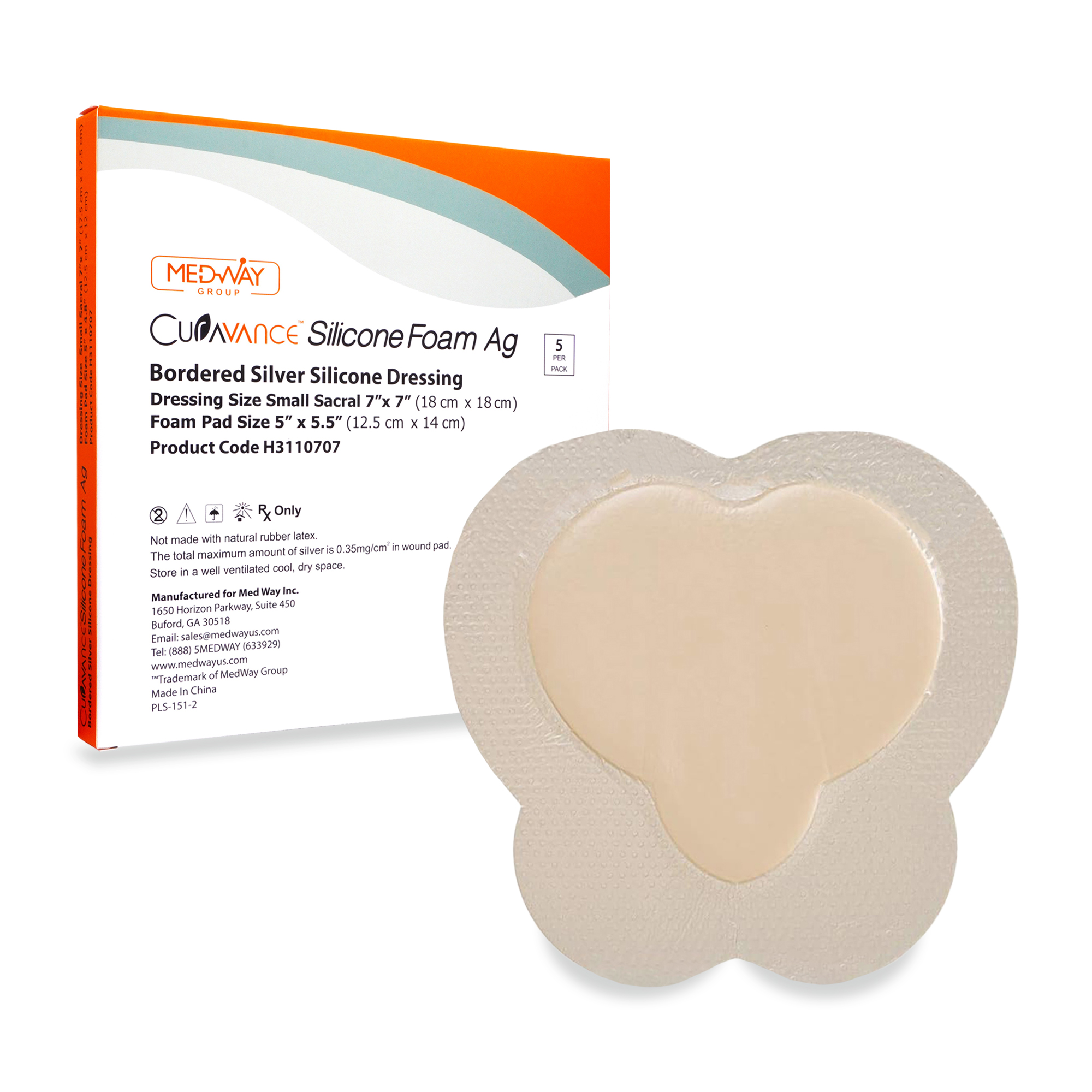
CuraVance® Silicone Foam Ag Bordered Dressings
Curavance® Silicone Ag Foam Dressings are intended for the management of moderate to heavily exuding acute and chronic wounds, including partial and full thickness wounds; such as leg ulcers.
CuraVance® Foam Ag Dressings
CuraVance® Foam Ag Dressings are multi-layer, non-adherent, antibacterial foam dressings with silver, comprised of moisture vapor permeable waterproof backing, absorbent foam and a soft wound contact surface
Nano Silver Wound Care
CuraVance® Nano Silver Gel
SiloVance Antibacterial Wound Dressing Gel is a water-based gel for use in moist wound care management. SiloVance Antibacterial Wound Dressing Gel contains silver that may help inhibit the growth of microorganisms within the dressing.
CuraVance® Silver Wound Wash
CuraVance® Silver Wound Wash aids in the removal of excessive secretion, dirt, and debris.
Collagen Dressings
MedVance® Collagen Dressings
MedVance® Collagen Dressings are 100% non-hydrolyzed type 1 bovine native collagen.
Proprietary technology process protects and retains native triple helical protein structure, allowing superior stability of the molecule and scaffolding through all four phases of wound healing.
Foam Dressings
MedVance® Foam – Bordered
MedVance® Foam is intended for management of moderate to heavily exuding acute and chronic wounds, including partial and full thickness wounds; such as leg ulcers, diabetic foot ulcers, pressure ulcers, donor sites, postoperative wounds, skin abrasions and superficial and partial thickness burns.
MedVance® Foam – Non-Adherent
MedVance® Foam is intended for management of moderate to heavily exuding acute and chronic wounds, including partial and full thickness wounds; such as leg ulcers, diabetic foot ulcers, pressure ulcers, donor sites, postoperative wounds, skin abrasions and superficial and partial thickness burns.
CuraVance® Silicone Foam Ag Bordered Dressings
Curavance® Silicone Ag Foam Dressings are intended for the management of moderate to heavily exuding acute and chronic wounds, including partial and full thickness wounds; such as leg ulcers, diabetic foot ulcers, pressure ulcers, donor sites, postoperative wounds, skin abrasions and superficial and partial thickness burns.
CuraVance® Foam Ag Dressings
CuraVance® Foam Ag Dressings are indicated for the management of
moderate to heavily exuding, acute and chronic wounds including: diabetic foot ulcers, venous leg ulcers, pressure ulcers, graft and donor sites, trauma wounds, second-degree burns, and post-operative wounds and skin abrasions.
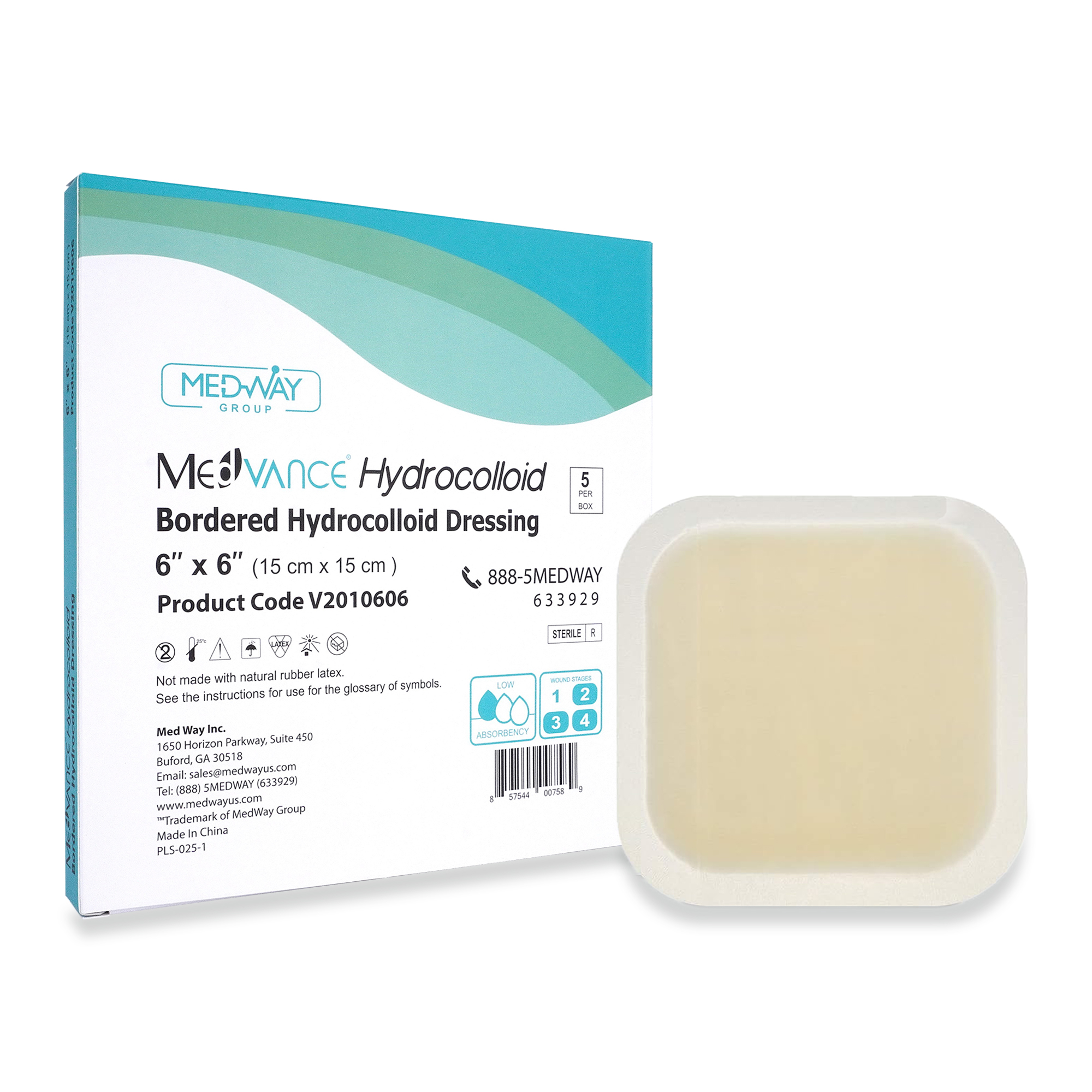
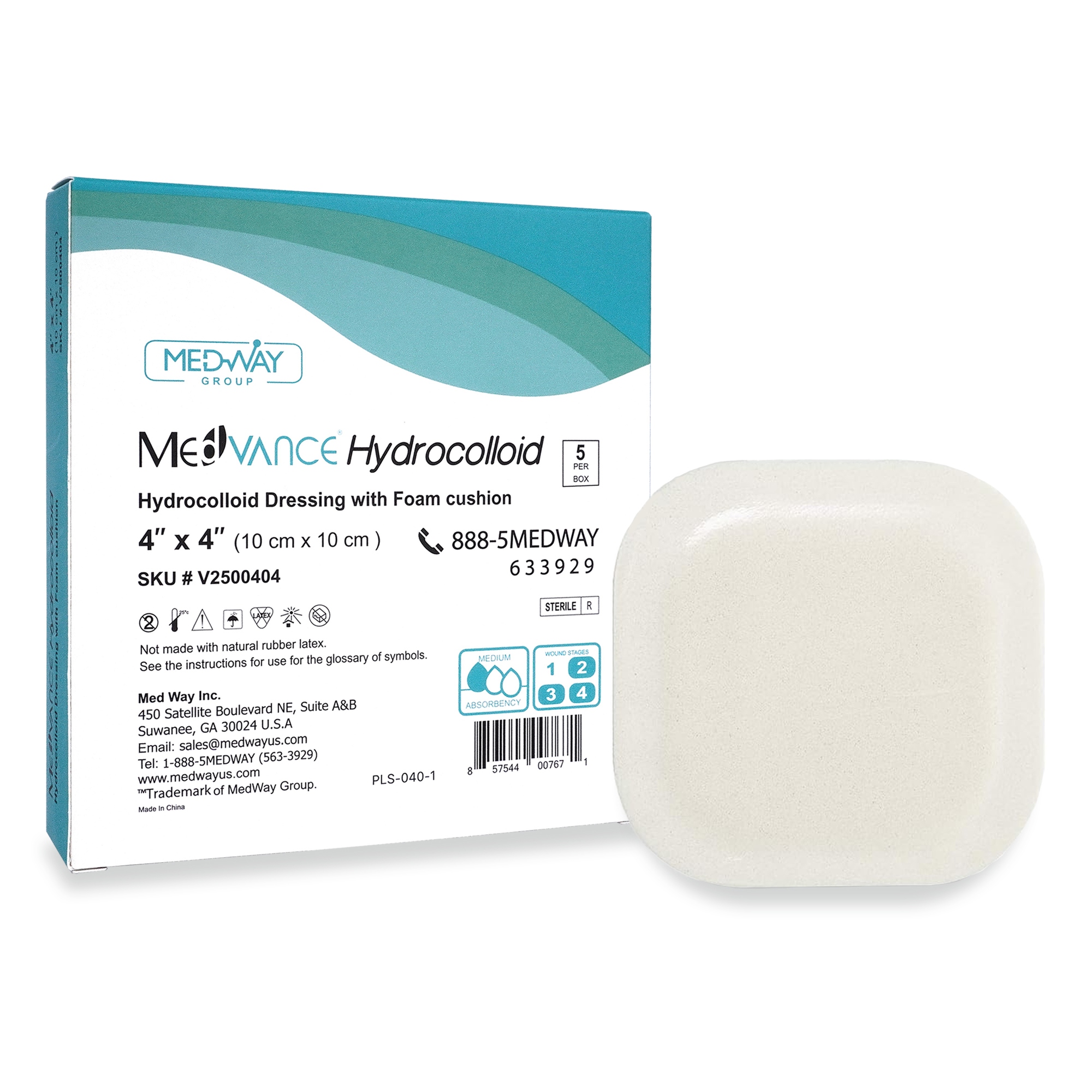
Hydrocolloid Dressings
MedVance® Hydrocolloid – Bordered
MedVance® Hydrocolloid Dressings are indicated for the management of light to moderately exuding wounds including venous leg ulcers, diabetic foot ulcers, pressure ulcers, superficial partial – thickness burns, donor sites, postoperative wounds and skin abrasions. Hydrocolloid dressings provide a moist wound environment that supports healing and autolytic debridement.
MedVance® Hydrocolloid – Thin
MedVance® Hydrocolloid Dressings are indicated for the management of light to moderately exuding wounds including venous leg ulcers, diabetic foot ulcers, pressure ulcers, superficial partial – thickness burns, donor sites, postoperative wounds and skin abrasions. Hydrocolloid dressings provide a moist wound environment that supports healing and autolytic debridement.
MedVance® Hydrocolloid – Cushioned
MedVance® Hydrocolloid Dressings are indicated for the management of light to moderately exuding wounds including venous leg ulcers, diabetic foot ulcers, pressure ulcers, superficial partial – thickness burns, donor sites, postoperative wounds and skin abrasions. Hydrocolloid dressings provide a moist wound environment that supports healing and autolytic debridement.
Silicone Dressings
MedVance® Silicone – Bordered
MedVance® Silicone Foam Dressings are intended for the management of moderate to heavily exuding acute and chronic wounds, including partial- and full-thickness wounds such as pressure ulcers, venous stasis ulcers, diabetic foot ulcers, traumatic wounds, abrasions and wounds healing by secondary intention.
MedVance® Silicone – Non-Bordered
MedVance® Silicone Foam Dressings are intended for the management of moderate to heavily exuding acute and chronic wounds, including partial- and full-thickness wounds such as pressure ulcers, venous stasis ulcers, diabetic foot ulcers, traumatic wounds, abrasions and wounds healing by secondary intention.
CuraVance® Silicone Foam Ag Bordered Dressings
CuraVance® Silicone Ag Foam Dressings are intended for the management of moderate to heavily exuding acute and chronic wounds, including partial and full thickness wounds; such as leg ulcers, diabetic foot ulcers, pressure ulcers, donor sites, postoperative wounds, skin abrasions and superficial and partial thickness burns.
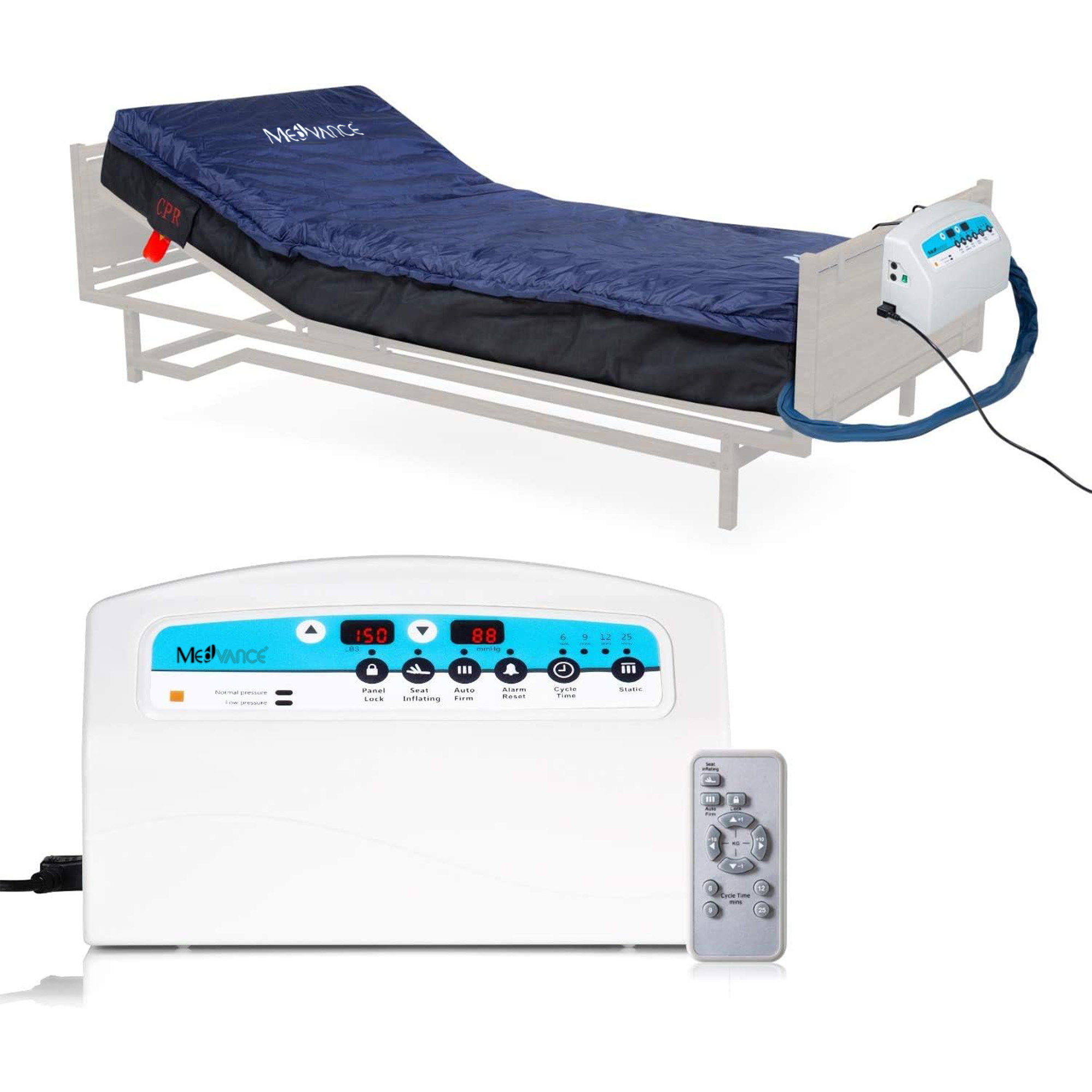
Support Surfaces
MedVance® Elite
The MedVance® Elite Dual Therapy Mattress is indicated for the prevention and treatment of pressure injuries / pressure ulcers.
MedVance® Comfort Mattress
The MedVance® Comfort Alternating Air Pressure Mattress Pad with Ultra Quiet Alternating Pump (Mattress & Pump only) | Pressure Sore/Ulcer Prevention and Relief | Use on Medical, Hospital, or Standard Bed.
MedVance® Eco Mattress
The MedVance® Comfort Alternating Air Pressure Mattress Pad with Ultra Quiet Alternating Pump (Mattress & Pump only) | Pressure Sore/Ulcer Prevention and Relief | Use on Medical, Hospital, or Standard Bed
MedVance® Elite with Integrated Cavity
The MedVance® Elite Dual Therapy Mattress with Integrated Cavity is indicated for the prevention and treatment of pressure injuries / pressure ulcers. A specialized recessed structure enhances comfort and alleviates pressure on the coccyx region, especially in seated positions that are elevated or upright.