Products
Alginates
Antibacterial Dressings
Foam Dressings
Hydrocolloid Dressings
NPWT
Silicone Dressings
Support Surfaces
Nano Silver
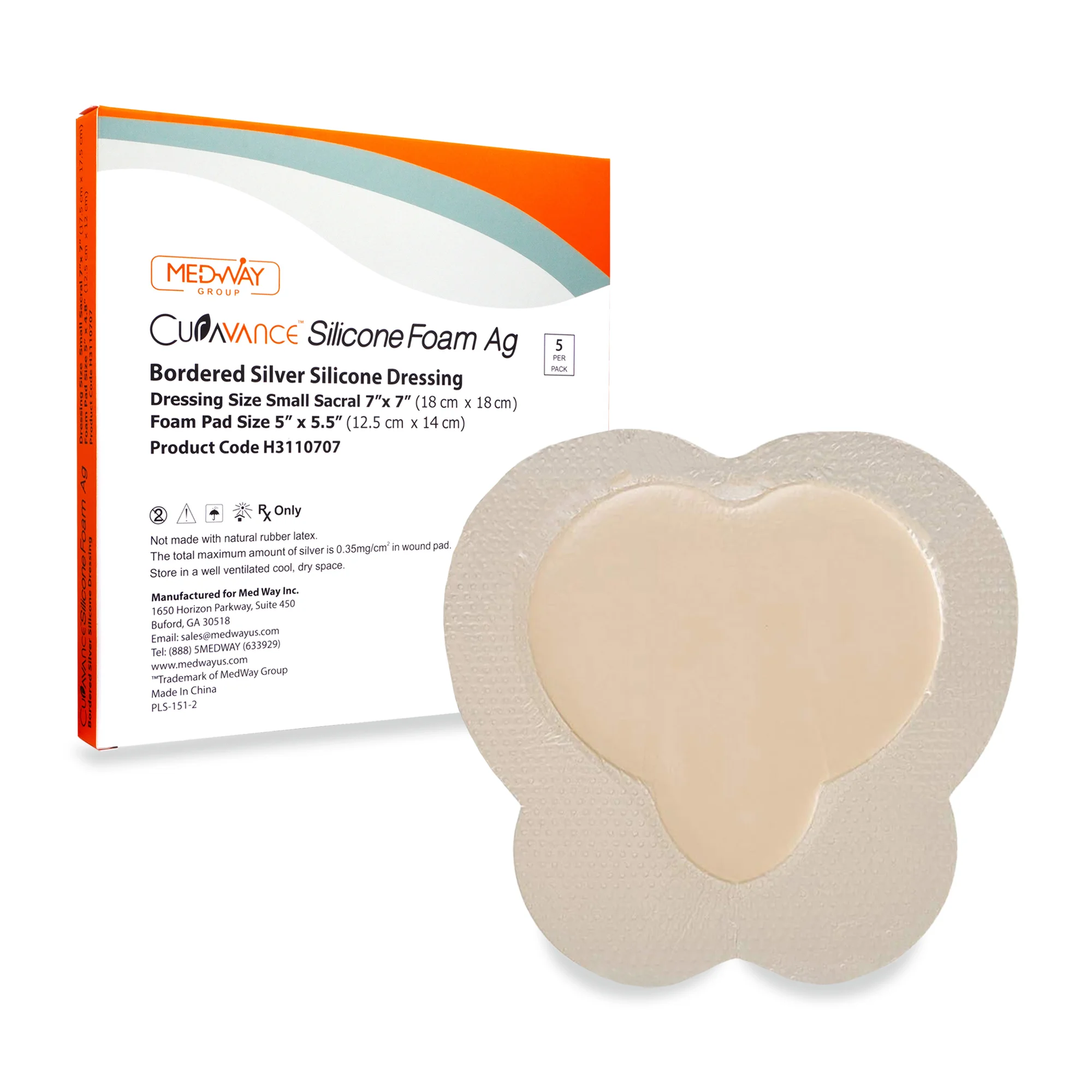
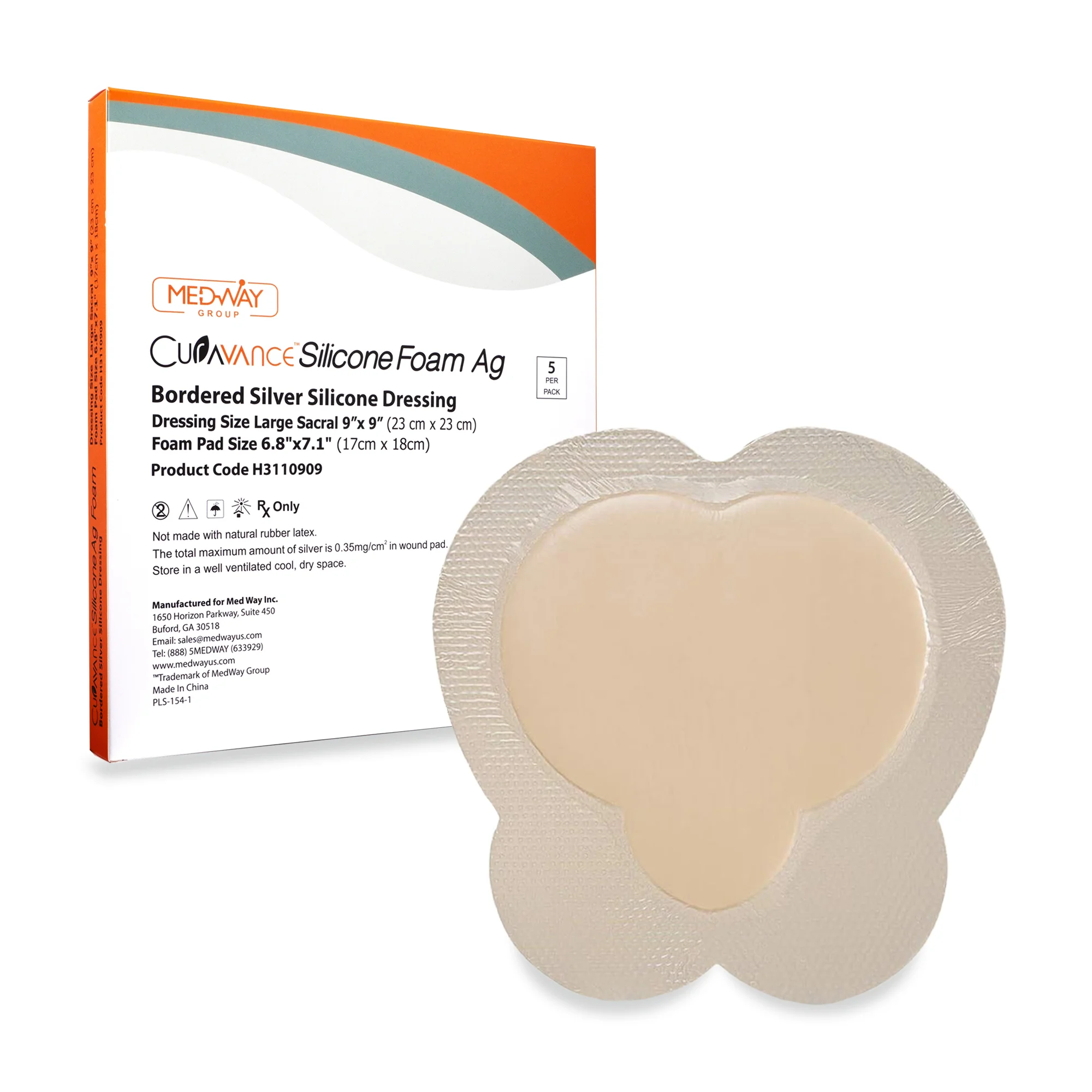
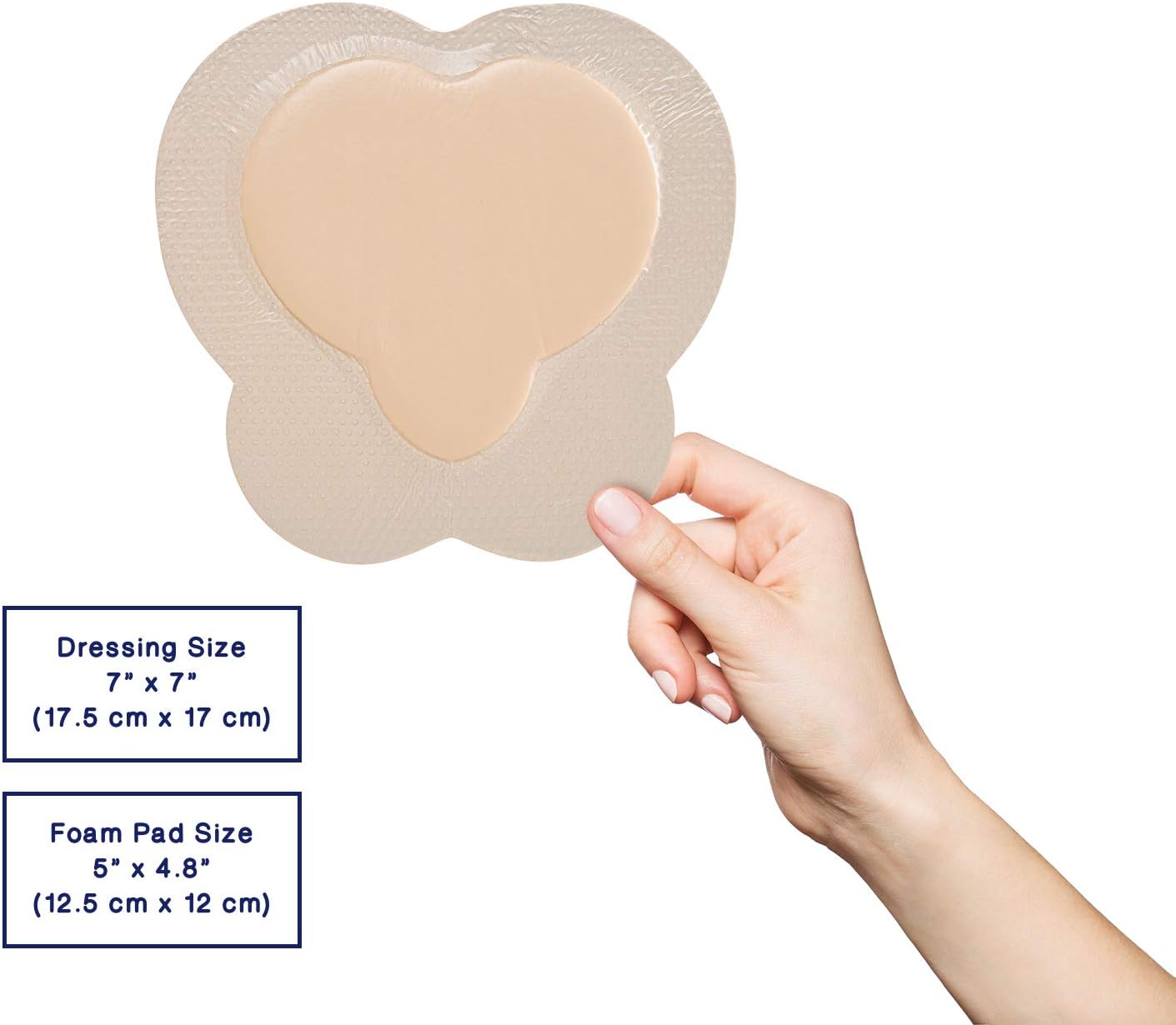
CuraVance® Silicone Foam Ag Bordered Dressings
Curavance® Silicone Ag Foam Dressings are intended for the management of moderate to heavily exuding acute and chronic wounds, including partial and full thickness wounds; such as leg ulcers, diabetic foot ulcers, pressure ulcers, donor sites, postoperative wounds, skin abrasions and superficial and partial thickness burns.
CuraVance® Silicone Foam Ag Bordered Dressings
Curavance® Silicone Ag Foam Dressings are multi-layered, antibacterial foam dressings incorporating a moisture vapor permeable waterproof backing, absorbent Ag foam, a superabsorbent core, and a soft silicone skin and wound contact surface.
Dressing wear time is enhanced through the use of a superabsorbent layer and fluid transfer layer that ensure maximum use of absorbent capacity and retention of fluid under compression. A semipermeable polyurethane film outer layer acts as a liquid and microbial barrier to prevent both strikethrough and contamination while delivering balanced moist wound healing. Soft, silver impregnated foam absorbs and retains exudate while providing broad spectrum antibacterial activity for up to 7 days.
The soft silicone adhesive wound contact layer and border maintain patient comfort, avoid MARSI and provide pain free atraumatic removal.
- Ionic silver provides sustained, broad spectrum antibacterial activity against the most common clinical pathogens associated with wound infection.
- Soft, hypoallergenic silicone adhesive acts as a wound contact layer and gentle adhesive border that sticks to surrounding intact skin but not to the wound bed, preventing secondary damage and minimizing discomfort during dressing removal and change.
- Highly absorbent core and distribution layer manage moderate to high amounts of exudate while providing a moist wound healing environment.
- Semi-permeable, polyurethane outer layer acts as a liquid barrier, while allowing the transmission of oxygen and water vapor to maintain a moist wound environment.
- Conforms to body contours and provides maximum patient comfort.
| Product Code | Dressing Size / Description | Pad Size | Packaging | HCPCS |
|---|---|---|---|---|
| H3010404 | 4″ x 4″ | 2″ x 2″ | 5 pcs/bx, 80 boxes/case | A6212 |
| H3110707 Sacral Small | 7” x 7” | 5″ x 5″ | 5 pcs/bx, 40 boxes/case | A6213 |
| H3110909 Sacral Large | 9” x 9” | 7″ x 7″ | 5 pcs/bx, 40 boxes/case | A6214 |
- Follow wound bed preparation protocol including wound cleansing and debridement.
- Select a suitable dressing pad size that overlaps the wound margin by approximately two centimeters.
- Apply pad to the wound bed and smooth down the dressing edge or adhesive border.
- To remove gently release dressing edge or adhesive border and remove dressing. Dispose as medical waste.
- Follow wound bed preparation protocol including cleansing with appropriate solution before applying subsequent dressings.
![]()